Understanding How Soil pH Affects Landscaping
Do you know how to achieve optimal growing conditions for your plants? It all has to do with the soil pH levels. The term “neutral soil” refers to soil with a balanced pH level. But how do you get a balanced pH level, and why is it important? Understanding how soil pH affects landscaping can ensure your plants and lawn stay healthy.
The Basics of Soil pH
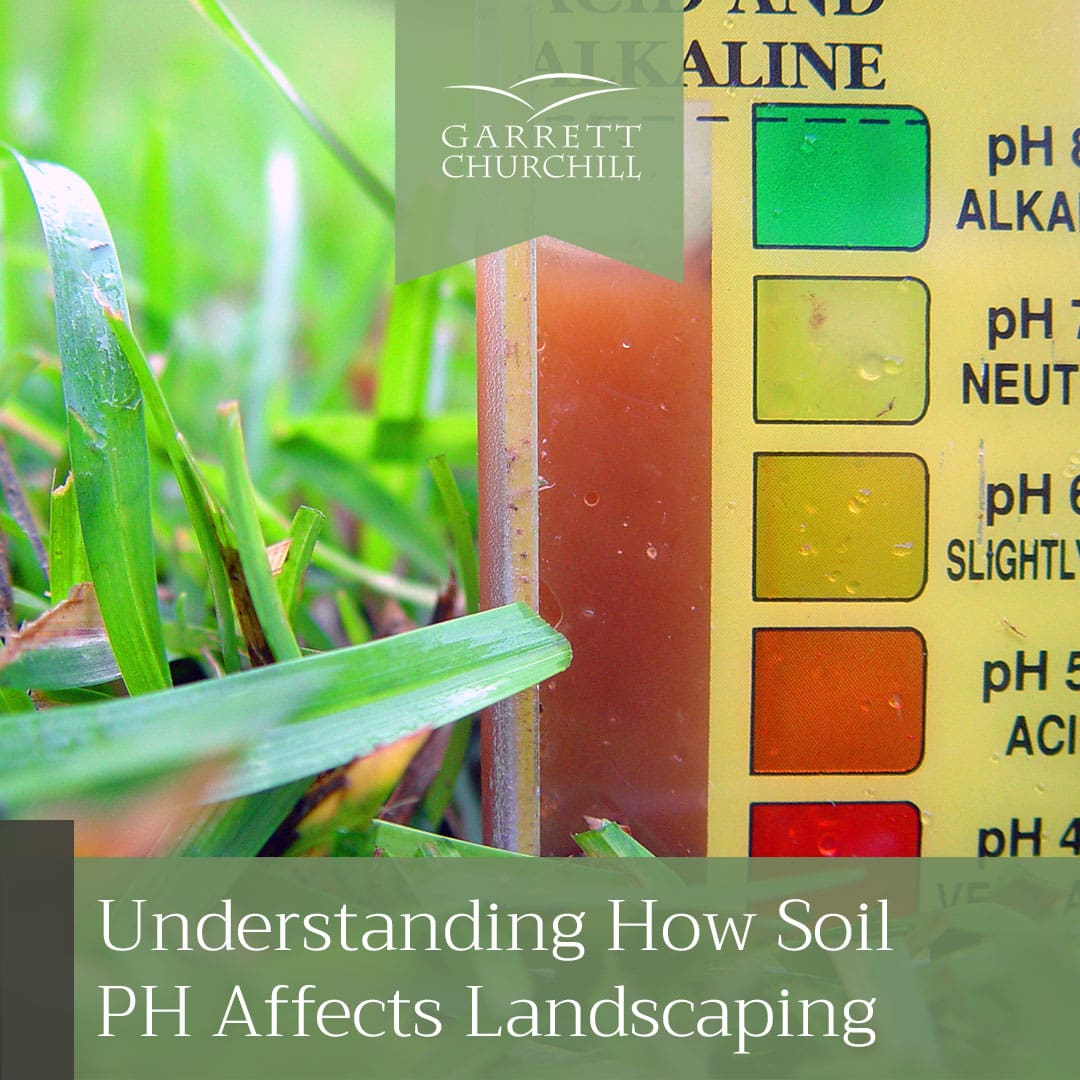
The potential hydrogen (pH) of soil is an indicator used to measure the alkalinity or acidity in a particular area. It is measured on a scale that goes from 0 to 14.
A soil pH level of 7 indicates the soil is neutral. Anything below 7 is acidic, and anything above is alkaline. Different plants prefer different levels of acidity and alkalinity, and not meeting these levels can result in a number of problems that can impact the growth of anything growing in it.
The Differences Between Acidic and Alkaline Soil
The differences between acidic and alkaline soil are more than just numbers on a scale. Each contains certain elements and minerals that can affect whatever grows there in a variety of ways.
Acidic Soil
Acidic soil occurs in situations where water has washed away most of the soil’s nutrients, namely calcium and magnesium. Once the soil becomes acidic, it harbors higher amounts of carbon dioxide, nitric acid, and even sulfuric acid.
Excessively acidic soil can be caused by a few different things, including decomposition and leaching. The use of chemical fertilizers and pesticides can offset the soil’s pH level to the extent that it becomes too acidic. Additionally, soil can become acidic due to heavy rains and acid rain, which wash away essential nutrients.
Certain perennials, such as perennials like rhododendron, azalea, and holly, thrive in acidic soil. Much like shrubs and trees, it’s best to research soil preferences before selecting the best perennials for your yard.
Alkaline Soil
Where acidic soil suffers from a lack of nutrients, alkaline soil suffers from the opposite problem. Alkaline soil contains an overabundance of calcium, magnesium, and sodium. In landscaping, it is often referred to as “sweet” soil.
This results in the nutrients being less soluble and therefore, less available to the plants.
Determining Soil pH
Determining your soil’s pH level is relatively easy. Simply collect a sample and send it to a soil testing lab. After you receive the results, you will be able to determine which plants are optimal for your soil. Depending on where you live, you might have to adjust your plant selection to alkaline or acidic soil.
Many landscaping plants, and even some grasses, are suitable for a wide range of soil pH levels. However, there are some plants that will not grow in one or the other, so make sure you plan effectively before expending resources.
If you are landscaping in Pennsylvania, the Pennsylvania Department of Conservation and Natural Resources provides recommendations on native plant selection.
Altering Soil pH
When dealing with soil pH, experts advise selecting plants that are well-suited for the soil in the area as opposed to using soil additives. This is because soil additives only work for a brief amount of time and require constant reapplication.
However, if you are certain that changing your soil’s pH level is the way to go, there are a few things you can do:
Raising the pH
Soil pH levels can be raised by adding a material with liming properties, such as dolomite or calcium carbonate. Always make sure to have your soil tested before applying any pH-altering materials.
If the soil in question is too acidic, you may need to perform a lime requirement test. This will measure your soil’s ability to resist pH changes.
Lowering the pH
If you are not in a hurry to lower the pH level of your soil, you can try adding elemental sulfur, which can slowly neutralize the alkalinity over the duration of several months, depending on your soil’s condition.
For a quicker solution, aluminum sulfate can immediately lower soil pH when it dissolves into the soil. However, you will need much more aluminum sulfate to accomplish this than you would elemental sulfur.
Get a Professional Soil Analysis Today
Are you wondering what your soil’s pH levels are? Our team has the years of experience necessary to provide you with accurate results to help your lawn and plants thrive. For your own professional soil analysis, contact Garrett Churchill today at 215.657.9160 or info@garrettchurchill.com.